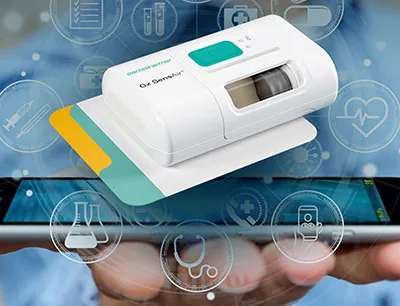
Gerresheimer and Aptar Digital Health, an expert in ‘software as a medical device’ (SaMD), digital patient support programs (PSPs) and disease management solutions, collaborate to develop an integrated solution for cancer therapy management. As part of this collaboration, the “Gx SensAir”-on-body drug delivery device will be connected to Aptar Digital Health's software-as-a-medical device platform to improve the treatment experience and outcomes of cancer patients. “Gx SensAir” can be used to administer large molecule biopharmaceuticals subcutaneously. The integrated solution aims to facilitate the onboarding of patients to new therapies, accompany them and help them better manage adverse effects, and make it easier to monitor patients remotely, ultimately improving treatment adherence and clinical outcomes.
“Combining our efforts opens up new exciting possibilities for optimizing the respective drug therapy and improving the quality of life for cancer patients”, said Daniel Diezi, Vice President Digitalization & New Business Models at Gerresheimer. “This collaboration in oncology will lay the groundwork for Aptar Digital Health and Gerresheimer to potentially expand into other therapeutic areas in the future.”
Gerresheimer and Aptar Digital Health combine expertise in device and software engineering
“We are honored to collaborate with Gerresheimer, a global industrial partner in drug delivery devices,” says Sai Shankar, President of Aptar Digital Health. “By combining our collective and proprietary expertise in device and software engineering, we believe this collaboration has the potential to deliver innovative solutions for patients, healthcare providers and the healthcare industry.”
Support and accompaniment of immunotherapies
Both companies believe that patient experience in oncology can be improved with new subcutaneous injection devices and digital platforms. For pharmaceutical companies, this collaboration is an opportunity to offer a patient-centric solution, and tackle challenges such as treatment interruptions or discontinuations related to administration issues or adverse effects. It also supports the transition from intravenous to subcutaneous administration of oncology drugs. The integrated solution will first be designed for targeted anticancer therapies such as PD-1/PDL-1, CTLA-4, with the potential to expand to all therapies delivered subcutaneously.









