In today’s competitive economy, some of the biggest challenges for pharmaceutical manufacturers are to increase availability, enhance performance, and improve product quality. In addition, companies need to optimize batch processing, which presents unique challenges not found in other manufacturing processes. One effective approach to address these issues is to utilize key performance indicators (KPIs) like Overall Equipment Effectiveness (OEE) to identify and close productivity gaps in batch processes. Operator efficiency is key to accomplishing these objectives, as operators are responsible for monitoring multiple stations through the facility, making sure that sequences in complex production lines of this highly regulated, batch-oriented industry proceed properly.
Through all of these functions, uninterrupted sequences and processes are the end goal. Any variation or deviation from standard operating procedures (SOP) affects the process and possible delivery and use of the product, which is detrimental in an industry where proof of consistency is critical. In many cases, operators today don’t have a clear view of the past, present and future of the batch procedures or the intelligent tools to identify the root cause of the variation or deviation. This can result in process inefficiency, can increase operator stress and can impact the quality of the final product.
Companies can address batch processing inefficiencies by gathering OEE data, and using that data as the foundation for dashboards that provide the operator with critical information, such as historical data, real-time visibility running batch processes, alerts in the event of problems, and access a look-ahead scheduler that helps the operator identify what needs to be done next.
All of this information can be presented on a single view, whether on a desktop, a mobile device or even a hands-free headset. Building on top of that foundation of OEE data, companies can apply automation, visualization, simulations, mobility and data analytics to improve batch process efficiency.
OEE can help optimize batch processes
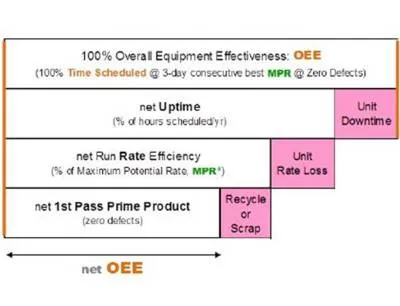
OEE measures manufacturing effectiveness at the operating unit level, helping companies determine what impact the current performance of any piece of equipment has on the overall efficiency of the manufacturing plant. OEE can help pharmaceutical companies focus on KPIs such as throughput or downtime and compare actual performance versus targets.
In batch processing, an OEE value is determined by measuring three variables: availability, performance and quality. Availability is a function of run time, which is negatively impacted by downtime; performance is measured by how close to maximum possible speed the system is running; and quality is based on how well products meet quality standards on the first pass. According to ARC, benchmarks for OEE vary by industry and type of manufacturing; however, world class performance generally falls in the range of an 85 percent OEE, although most companies struggle to reach 60 percent.
But OEE is more than just a number, OEE data can be leveraged to help companies improve operating performance in a number of ways. OEE provides automatic visibility into batch processes, helping companies track downtime events and identify the cause for the downtime event so companies can pre-emptively make fixes, track bottlenecks and gauge overall cycle time for each process. Armed with this information, operators and maintenance workers can gain a better understanding of where to focus their efforts. And companies can use positive OEE metrics to help win new business.
New ways to improve batch processes
OEE can help pharmaceutical companies gain a competitive advantage by improving the performance of their assets. With the data that OEE provides, plants can increase operation availability and process performance, while ensuring visibility for product quality. On the other hand, by failing to measure OEE, companies risk overlooking performance improvement opportunities and falling behind competitors.
An important part of implementing an OEE strategy is providing an operator user interface with visualization technology that automatically constructs look-ahead views so operators can easily access insights to upcoming events or potential delays in the batch process. This look-ahead workflow can then be used to provide the flexibility to conduct more tasks concurrently.
The key to OEE is guided troubleshooting, provided to help operators keep the batch progressing. The latest technologies facilitate guided troubleshooting through clear, intuitive displays illustrating batch sequences in an understandable manner. This allows operators to better anticipate and respond to process deviations or equipment failures. Ultimately, this analysis can uncover best practices that can be deployed across all batches, decreasing cycle time and improving productivity. Visual analytics also reduce stress and enable operators to take on parallel tasks with confidence, knowing that there is sufficient time before the next action is required.
New technologies focused on OEE also enable companies to improve operational efficiency of batch processes in other ways. For example, companies can replace a centralized batch engine running on a server with a distributed set of redundant modular controllers. A controller-based system provides redundancy, robustness, and increased throughput by reducing execution times and latency between applications levels.
Companies can use enhanced simulation capabilities to reduce the time and effort required for testing and validation. In the past, moving to a new or modified configuration from a lab environment to real-time could take hours of validation and documentation. By downloading the new configuration directly to the batch controller, companies can move from test to production effortlessly. These new technologies make it much easier for pharmaceutical manufacturers to implement OEE KPIs in order to increase availability, boost performance and improve quality in batch processing.
Conclusion
Pharmaceutical companies are under pressure to increase efficiency and reduce costs. Operators are being asked to squeeze more productivity out of plant assets, to be more effective at troubleshooting batch processes, and to identify new ways to be more productive, more efficient and to produce higher quality products.
Utilizing OEE can provide an important competitive advantage, if properly implemented. Advanced visualization technology allows OEE data to be aggregated and analyzed, enabling companies to identify best practices that can be applied across all batches, decreasing cycle time and improving overall productivity and quality.
Looking ahead to future advances, the advent of operational mobility and intelligent analysis will support feeding OEE data into emerging data analytics systems that use machine learning and artificial intelligence to spot trends and identify innovative ways to improve process efficiency even more. These advances can be applied across all of the company’s batch processing facilities to enable even greater improvements in performance, availability and quality.
Author: Steve Zarichniak, Honeywell Technical Solutions Consultant